Heat
There are three ways in which heat can flow:
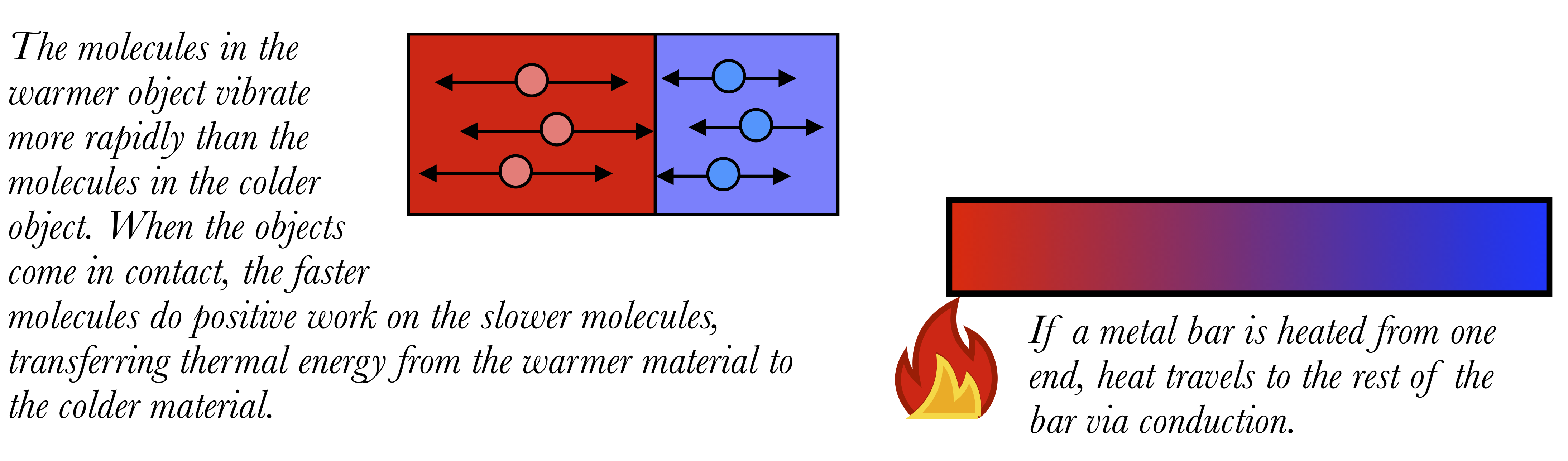
Conduction
Conduction occurs within an object or between two objects which are in contact with each other. It can occur in solids or fluids.
Convection
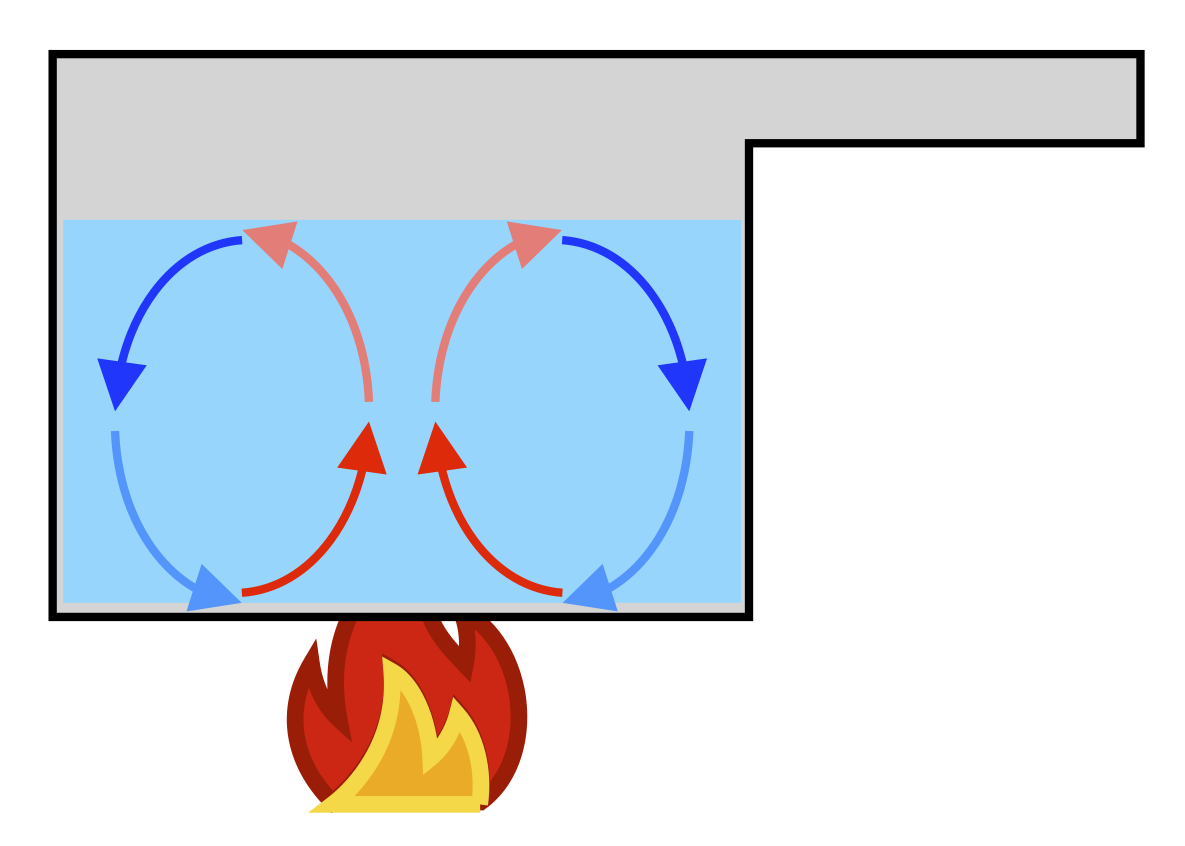
Convection occurs within a fluid such as air or water, where warm parts of the fluid flow towards colder places and vice versa: heat is carried directly from one region to the other by molecules, instead of passed from molecule to molecule as in conduction.
For example, hot air is less dense and so tends to rise while colder air sinks: this creates convection rolls which can warm a room more easily or, on a larger scale, the Earth’s atmosphere (where convection causes winds and weather).
Radiation
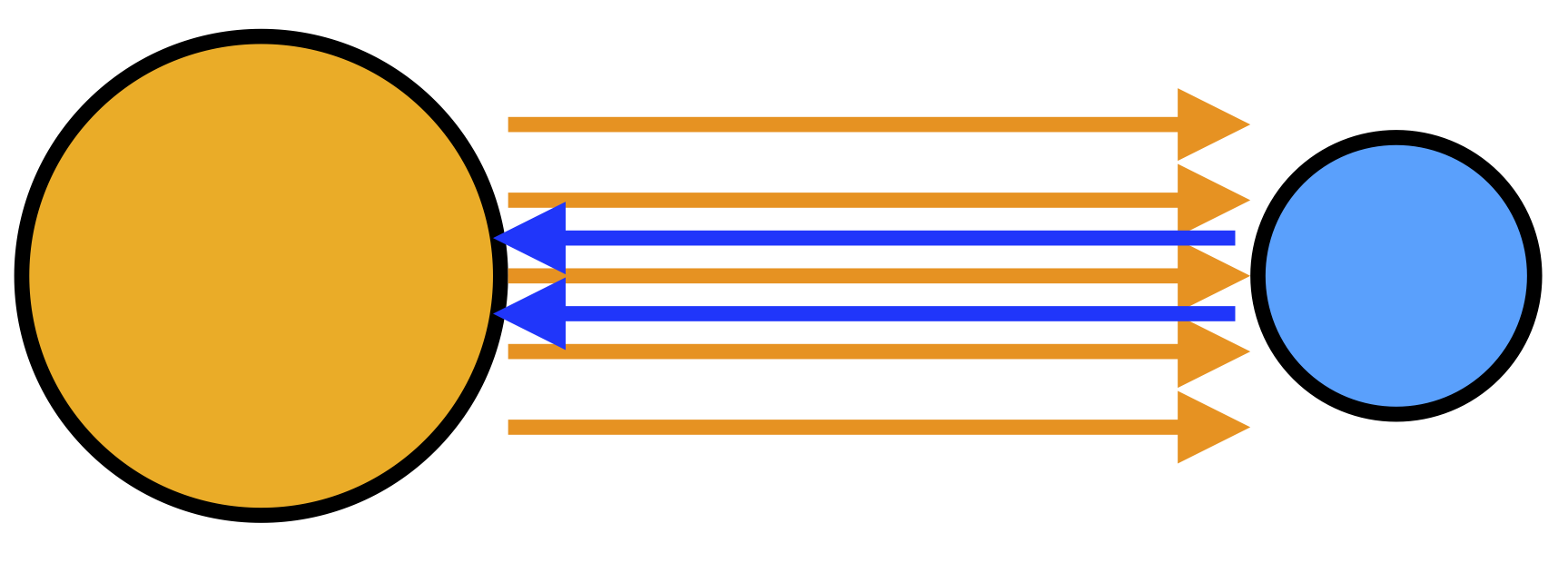
All objects radiate electromagnetic radiation depending on their temperature: mostly infrared light, although hotter objects (like molten iron, for instance) radiate visible light as well. For example, the Sun and the Earth both radiate energy and absorb each other’s energy as well, but the Earth absorbs more radiation from the Sun than vice versa, and so heat is transferred from the hotter Sun to the cooler Earth. Radiation is the only type of heat which can travel through a vacuum, although it can travel through other media as well. If you’ve ever felt a warm fire or the warm Sun when the air is cold in winter, you’ve felt their radiated heat.