Temperature
Some Useful Temperatures
| Reference | Celsius | Kelvin | Fahrenheit |
| absolute zero | -273°C | 0K | -459°F |
| water freezes | 0°C | 273K | 32°F |
| an autumn day | 10°C | 283K | 50°F |
| room temperature | 20°C | 293K | 68°F |
| warm summer day | 30°C | 303K | 86°F |
| body temperature | 37°C | 310K | 99°F |
| water boils | 100°C | 373K | 212°F |
You probably have an intuitive grasp of what temperature is already: hotter objects have higher temperatures than colder objects. At the microscopic level, temperature is the measure of the amount of random energy the atoms and molecules of a material have: as the temperature rises, the molecules in an object begin to vibrate (or in a fluid, rotate and wander about) more quickly. Temperature is related to thermal energy, except thermal energy also depends on size (a ton of bricks will have more thermal energy than a single brick with the same temperature) and material (water holds more thermal energy than iron, for instance).
The official SI unit of temperature is called the kelvin, and it is an absolute temperature scale: this means that the molecules in an object with a temperature of 0K (which is called absolute zero) have no thermal energy: they do not vibrate or rotate or wander at all. Absolute zero in Celsius is –273°C, so we can convert from Kelvin to Celsius by adding 273, and convert back by subtracting.
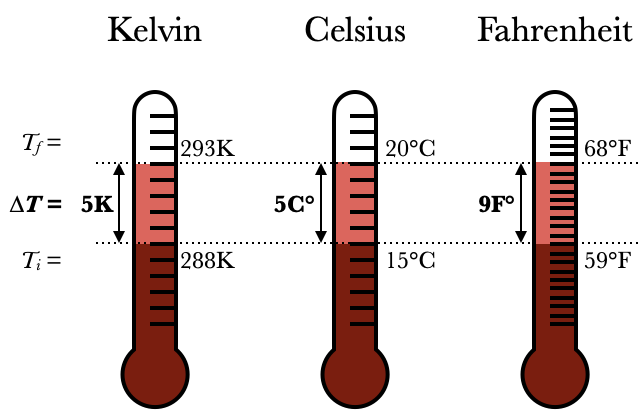
The Kelvin scale is defined so that the size of a Kelvin degree is equal to the size of a Celsius degree: that is, the distance between tics on the two thermometers are the same. (See the picture on the right.) This means that changes in temperature °T have the same value in Celsius and Kelvin, and even though individual temperatures are not. For instance, the thermometers on the right show an increase in temperature from 15°C to 20°C, which is also an increase in temperature from 288K to 293K. But both thermometers show an increase of 5. To make the distinction, we write 5°C ("five degrees Celsius") to indicate a specific temperature on the thermometer, but 5C° ("five Celsius degrees") to indicate a change in temperature. Note that
(Fahrenheit has a smaller spacing in between its degrees, so a change of 5C° corresponds to 9F°)
As a rule of thumb:
When a physics equation contains a