Heat Engines
A heat engine is a device that converts thermal energy into usable energy. They typically work by allowing heat to flow into or out of a working substance such as air or water: the working substance expands or contracts due to the change of temperature, and this allows the substance to do work on the outside world. For example, in a steam engine, water (in liquid and gas form) is the working substance. It is converted into steam due to an external energy source (burning fuel, concentrated sunlight, or nuclear fission, for example), and the flow of heat out of the steam is converted into useful work, whether mechanical, chemical, electrical, or some other type. An internal combustion engine in a car uses an air-fuel mixture to do the same, although in this case the source of heat is the ignited working substance itself, hence the name internal combustion. Heat engines are in fact where we get most of our useful energy.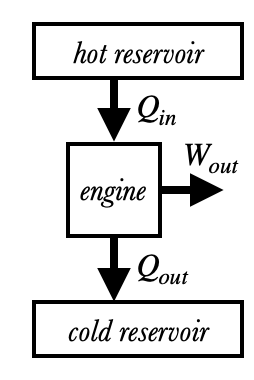
This figure shows a simplified diagram of a heat engine’s operation. Heat $Q_{in}$ flows into the engine from a hot reservoir, something which is warmer than the engine itself (like burning gasoline or steam). Some of that heat is then output as work $W_{out}$ to be converted into kinetic, potential, electrical, or another type of useful energy. The remainder of the heat $Q_{out}$ (called waste heat or exhaust) is then expelled into a cold reservoir, which must be colder than the engine. (This is normally the surrounding air or water.) The relationship between the three energy flows is
These energy flows can either be measured in joules (if we're talking about the total energy flows) or watts (the rate of energy flow). Note that all three quantities are considered to be positive despite their having different directions.
which is a number between 0% and 100%. A 25% efficient engine ($\eta=0.25$), for instance, will convert one-quarter of the heat input into work and dump the remaining three-quarters as waste heat.