Positive and Negative
The ancient Greeks were aware that amber rubbed with fur would attract straw and other light objects, and that they could even get sparks from the amber if they rubbed it long enough. In 1600, William Gerbert coined the term electrica to describe this effect, from the Greek word for amber, "ἤλεκτρον" or "ēlektron". Experiments in electricity continued through the 1600s and 1700s by many scientists, including the American Benjamin Franklin (who was so famous for his experiments in Europe that he was treated like a superstar when he came to France to plead the colonies' case). Other scientists had discovered that electricity came in two forms, but Benjamin Franklin developed a "one-fluid theory of electricity", suggesting that electricity came in two types, "positive" and "negative", which were able to cancel each other out.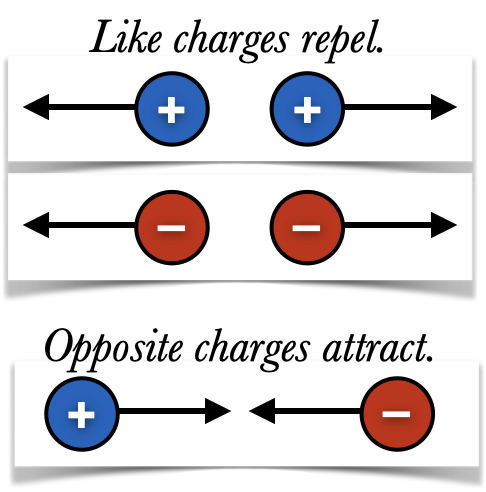
We now know that Franklin was largely correct: there are two types of charges, positive and negative, which exert forces on one another:
Charges with opposite signs attract one another.
Or in a more pithy form, "Like charges repel, opposites attract."
The SI unit of charge is the Coulomb, abbreviated C, and the variable we use is either $q$ or $Q$. (Unlike most variables, the capitalization here can vary.) Coulombs are very large (as we will see ): most day-to-day charge is usually measured in millicoulombs ($1\u{mC}=10^{-3}\u{C}$), microcoulombs ($1\u{\mu C}=10^{-6}\u{C}$), nanocoulombs ($1\u{nC}=10^{-9}\u{C}$), or even smaller amounts. Thus a balloon that is negatively charged by rubbing it on a wool sweater might have a charge of $q=-4\u{\mu C}$, while the sweater might pick up a positive charge of $q=+4\u{\mu C}$.
Objects with an even balance of positive and negative charge are called neutral, and have $q=0$. Surprisingly perhaps, neutral objects can be attracted to both positive and negative objects, a phenomenon which we will discuss in Polarization.