Polarization
As I mentioned earlier, neutral objects can be attracted to both positive and negative charges. This is due to a phenomenon called polarization.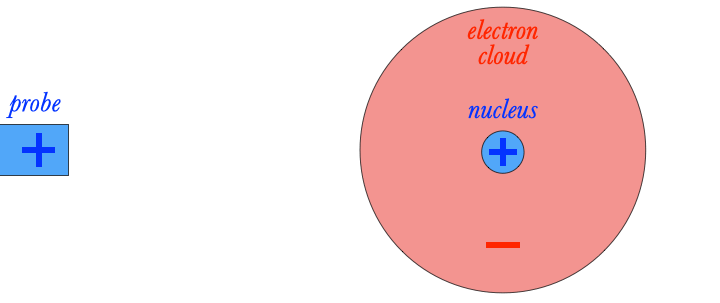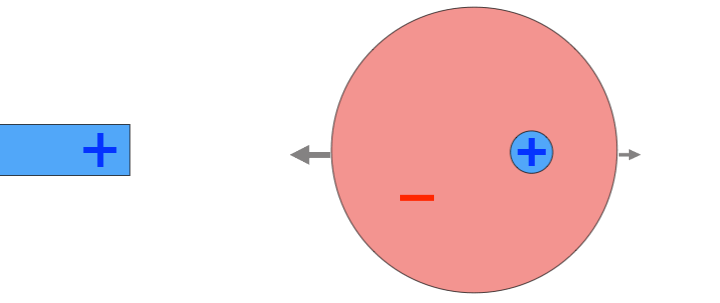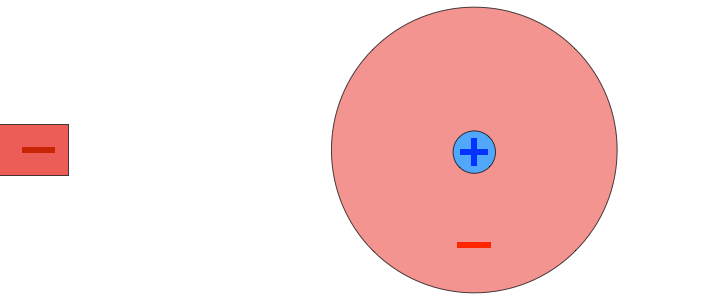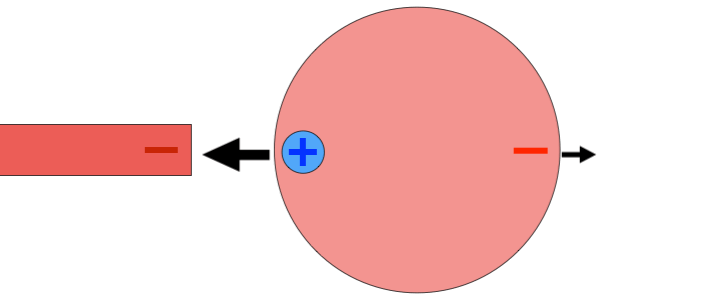
For example, suppose I place a positively charged probe next to an atom. The positively charged nucleus will be repelled by the probe, feeling a force to the right, but the negatively charged electron cloud will be attracted to the probe. As a result, the atom becomes polarized, with a positive side and a negative side. Because the negative side of the atom is closer to the positively charged probe, the atom feels a stronger attractive force than a repelling force. The result: the atom feels a net attraction towards the probe. If a negative probe had been used instead, the atom would have polarized in the opposite direction, but attraction would have occurred as well.
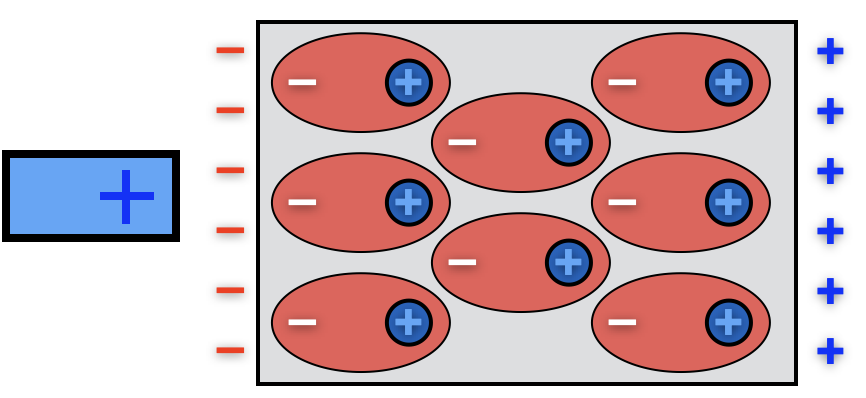
When a neutral object is brought near a positively-charged probe, the atoms in the object all polarize. In the interior of the object the charges cancel out, but the near surface develops a layer of negative charge, and the far surface a positive one. The object itself becomes polarized, and is attracted to the probe in the same way as the individual atoms are.
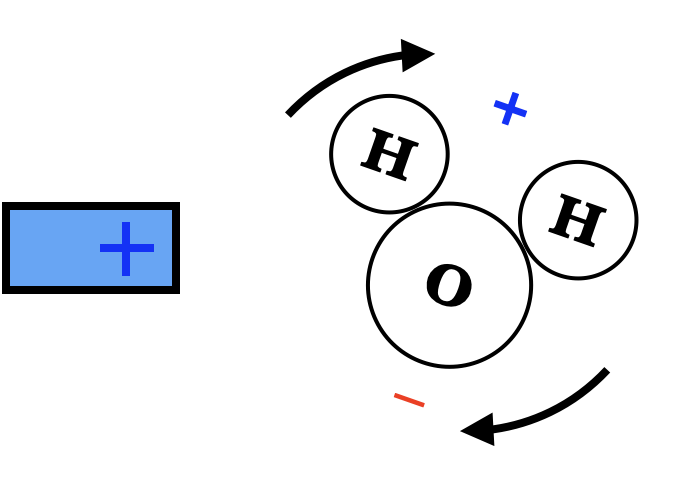
Some materials are better at polarizing than others are, particularly those materials made up of molecules which are naturally polarized. For instance, a water molecule is made up of one oxygen atom and two hydrogen atoms. These atoms share electrons, but because the oxygen's nucleus is larger, the extra electrons end up spending more time on the oxygen end, and so the hydrogen end of the molecule tends to be positive. A water molecule doesn't just stretch in the presence of a charged probe, it rotates, and because rotation is much easier than stretching, the water molecule feels a fairly strong attraction.
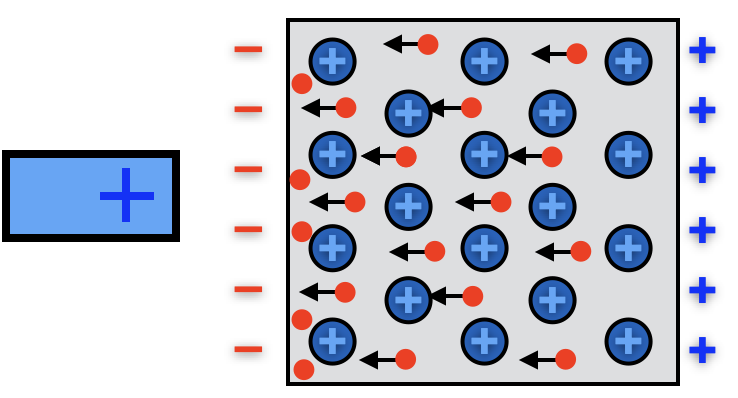
Metals are even better at polarizing, because in a metal every atom contributes one or more electrons to an "electron sea" which can move freely throughout the metal. When a positive probe is placed near a metal, electrons from all over the metal rush over to its side, resulting in a much stronger polarization effect.