Pressure
It doesn’t take a lot of force to cut into an orange, but it does depend on how that force is applied. Pressing on the skin of the orange with your open palm isn’t going to do much, but if we apply the same force with a knife, the knife cuts through the skin easily. This is because the knife applies the force to a smaller area of the skin, concentrating its efforts. The amount of force applied to a particular area on a surface is called pressure:
which is called atmospheric pressure, and sometimes written as "1 atm". Since the average human has a surface area of about $2\u{m^2}$, that means that the air is pushing on you with a total force of $$\begin{eqnarray} F&=&(1.01\times 10^5\mathrm{N/m^2})(2\mathrm{m^2})\\ &=&200,\!000\mathrm{N},\\ \end{eqnarray}$$ more than a hundred times larger than the weight of an average person (1500N).
But wait: if the force of the atmosphere is so large, why haven't we all been crushed? Partly it’s due to our skin and bones fighting the atmosphere, but mostly it’s because the fluids in our body are exerting the same pressure on us from the inside, in a constant “push-of-war” with the external atmosphere. Our body is careful to maintain this balance of pressures: if you travel in an airplane or into the mountains where the outside air pressure is lower, your ears will “pop” as the body releases some of its own internal pressure to compensate.
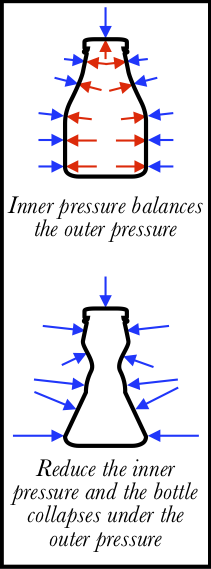
This same pressure balance occurs with any object. For example, a plastic soda bottle has air inside which exerts 1 atm of pressure outward, counteracting the 1 atm of pressure inward. If you suck some of the air out of the bottle, however, you reduce that internal pressure, and the external pressure of the air causes the bottle to partially collapse. We call this phenomenon suction. Your mouth isn’t pulling the walls of the bottle in; the outside air is pushing the walls in. The same thing happens when you drink through a straw: the entire atmosphere conspires to push the drink into your mouth. Pretty neat.