Power Flow
There are many instances where energy flows steadily into and out of a system, and in these instances the concept of power is particularly useful. For example, humans consume a certain amount of energy from food (usually measured in Calories) per day, and output a certain amount of energy in motion, sound, and (mostly) heat. "Calories per day" is a measure of power (energy divided by time) which is equal to about 0.05 watts: it doesn't usually make much sense to talk about the total energy consumed by a person because that depends on how old they are. Similarly, electrical devices are usually rated on the amount of electricity they require in watts because the manufacturer can't know how long you plan on running them.If the power input into a system is $P_{in}$, then during a time period $\Delta t$ the system gains energy equal to $\Delta E=+P_{in}\Delta t$. Conversely, the system loses energy due to power $P_{out}$ being output by the system, and so the change in the system's energy is
For example, if a person consumes more calories in a day than they output, then $P_{in}>P_{out}$ and the total energy stored in their body (as glucose, glycogen, or fat) is positive. When the power output is greater, the body uses up its energy stores and so $\Delta E<0$. (Which is where we get the traditional "calories in versus calories out" dieting advice, although overly simplistic due to how complex the power output of the human body is: in many cases decreasing $P_{in}$ will cause the body to reduce $P_{out}$ to avoid starving, resulting in an unexpected increase in $\Delta E$.)
There are some systems which are incapable of storing energy, such as the nationwide electrical grid. In cases like that, the power in must be equal to the power out. In the case of the electrical grid, power is constantly being routing from one place to another to meet demand or to use up excess supply without it becoming dissipated as heat: a malfunction in this routing system can lead to widespread blackouts. Switching electrical production from constant sources like fossil fuels or hydroelectric to variable sources like solar and wind will probably require the introduction of large-scale energy storage such as batteries, capacitors, or hydroelectricity.
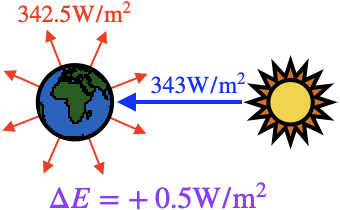
Other systems are able to store energy only by heating up, which can cause negative consequences for the system. The current-day Earth is probably the most important example of this. The Earth receives an average of 343 watts of power from the Sun for every square meter of its surface. (The Earth's surface area is 510 trillion square meters, so this is a lot of power.) In the past, the Earth radiated the same amount of energy as heat into space, and so the total energy of the Earth remained relatively constant. However, due to increased amounts of carbon dioxide and methane in the atmosphere blocking some of that radiation, only 342.5 watts of power (our best estimate as of 2021) are being radiated (on average) from every square meter, so that the Earth needs to store 0.5 watts per square meter, which is $2.2\times10^{19}\mathrm{J}$ every day. This excess energy is stored partially as increased wind speeds (stronger hurricanes) but mostly as thermal energy, raising the Earth's average temperature. Now as the Earth gets warmer, it will output more power into space, so that eventually the power input and output will reach equilibrium again, and the planet will be fine. Whether human life will be able to exist in that equilibrium state is another matter entirely.
Footnote
The calorie is a unit of energy which was introduced back when people thought thermal energy was different from other types of energy. One calorie is equal to 4.19 joules; however, when people talk about Calories in food they are actually referring to kilocalories, which are often written as Calories with a capital C. (Yes this is incredibly confusing.) If a person consumes "2000 Calories" during a day, that is equivalent to 2,000,000 calories or 8,380,000 joules worth of energy! That sounds like a lot, but if we spread it out over a day we find it isn't so ridiculous: $$2,\!000,\!000\u{cal/day}$$ $$\times {4.19\u{J}\over 1\u{cal}}\times {1\u{day}\over 86,\!400\u{s}}$$ $$=97\u {W}$$